Abstract
Background. Carfilzomib is a proteasome inhibitor, currently approved for use in relapse-refractory Multiple Myeloma (RRMM).1 There is mixed data on safety of Carfilzomib in patients (pts) with renal impairment (RI) and minimal in the upfront setting, partly due to exclusion of these pts from trials. Pooled data from phase 2 studies of single-agent Carfilzomib in RRMM, noted 24.1% acute kidney injury (AKI), including 23.8% of pts with moderate to severe renal dysfunction (CrCl<50 mL/min).2 Interestingly, severity of renal insufficiency did not have an effect on incidence of toxicity nor overall response rate.3 However, in FOCUS (single agent Carfilzomib vs Dexamethasone +/- Cyclophosphamide in RRMM), grouped renal events were three times more common in patients with baseline CrCl<30ml/min than those with CrCl>50 ml/min.4
We previously reported on 71 pts receiving induction Carfilzomib, whose baseline RI was associated with incidence of AKI. Of pts with normal renal function, 36% developed AKI, compared to 59% and 62.5% of those with mild and moderate impairment, respectively.5 Current management dictates dose reduction only once CrCl ≥ 15ml/min.6 The aim of this retrospective study is to report on the safety profile and efficacy of induction Carfilzomib in pts with RI of newly diagnosed MM as evidenced by proteinuria.
Methods. Patients were selected from the CarBiRd study (induction Carfilzomib and Dexamethasone; with subsequent Clarithromycin, Lenalidomide, and Dexamethasone; with Lenalidomide maintenance) at the Weill Cornell Medicine Myeloma Center. Pts were grouped based on renal involvement of myeloma. Baseline renal function was established by measuring pre-enrollment GFR using the Modification of Diet in Renal Disease (MDRD) equation. Subjects were stratified into 3 sub-groups: normal function (GFR>80 ml/min/1.732), mild impairment (GFR 50-80 ml/min/1.732), and moderate impairment (GFR 30-49 ml/min/1.732). Incidence of AKI was assessed during induction with Carfilzomib and Dexamethasone only. AKI was defined by increased serum creatinine (sCr) according to the National Cancer Institute's Common Terminology Criteria for Adverse Events Version 4.0.6 sCr and m-protein were measured prior to therapy and upon completion of Carfilzomib/dex induction. Overall response and toxicity were also assessed.
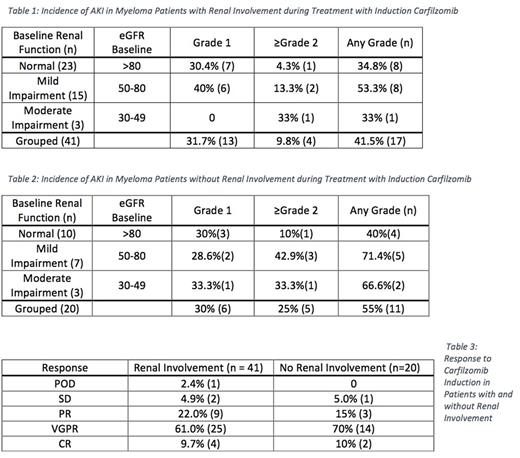
Results. 75 patients were enrolled in the CarBiRd study. One withdrew for fever and AKI. Of the 61 patients with evaluable urine m-protein, 23 had normal kidney function, 15 mild impairment, and 3 moderate impairment. AKI was seen in 34.8% of patients with normal renal function, 53.3% with mild and 33.3% with moderate impairment. Of the 17 patients who had AKI, 70.6% had a return sCr within 0.15 mg/dl of baseline. Mean number of cycles of Carfilzomib was 5 (range 3-12). The mean fall in m-protein at end Carfilzomib was 87.4%.
Of patients without proteinuria, 10 had normal kidney function, 7 had mild impairment, and 3 had moderate impairment. In pts with normal function 40% developed AKI, whereas those with mild and moderate impairment reported 71.4% and 66.6%, respectively. Mean number of cycles of Carfilzomib was 6.2 (range 3-14). Of the 11 patients who developed AKI, 91% had a return to serum creatinine within 0.15 mg/dl of baseline.
Discussion. Data on renal toxicity of carfilzomib in patients with renal involvement of myeloma are limited to date, confounded by exclusion of renal impairment from studies, data mainly in the RRMM setting where prior or concurrent drug exposure may play a role. Previous data indicate baseline renal impairment may predict AKI. We report on a cohort of pts with newly diagnosed myeloma who received carfilzomib/dexamethasone induction, where renal involvement did not predict increased toxicity. In addition, most cases of reported AKI resolved prior to next week dosing, and required no intervention beyond hydration. We acknowledge the small cohort and single-arm, single-center nature of the study. As seen previously, response to therapy does not appear to be affected by renal impairment. At this time, myeloma kidney and decreased GFR should not preclude use of carfilzomib in pts with myeloma.
Perry: celgene: Honoraria. Pekle: celgene: Honoraria; BMS: Honoraria. Niesvizky: BMS: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Amgen: Consultancy. Rossi: Thrassos: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal